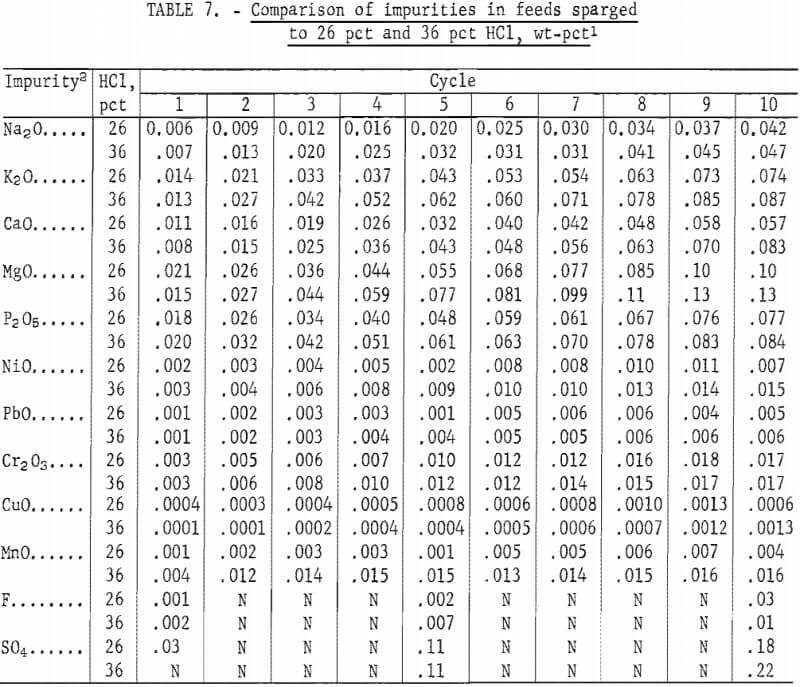
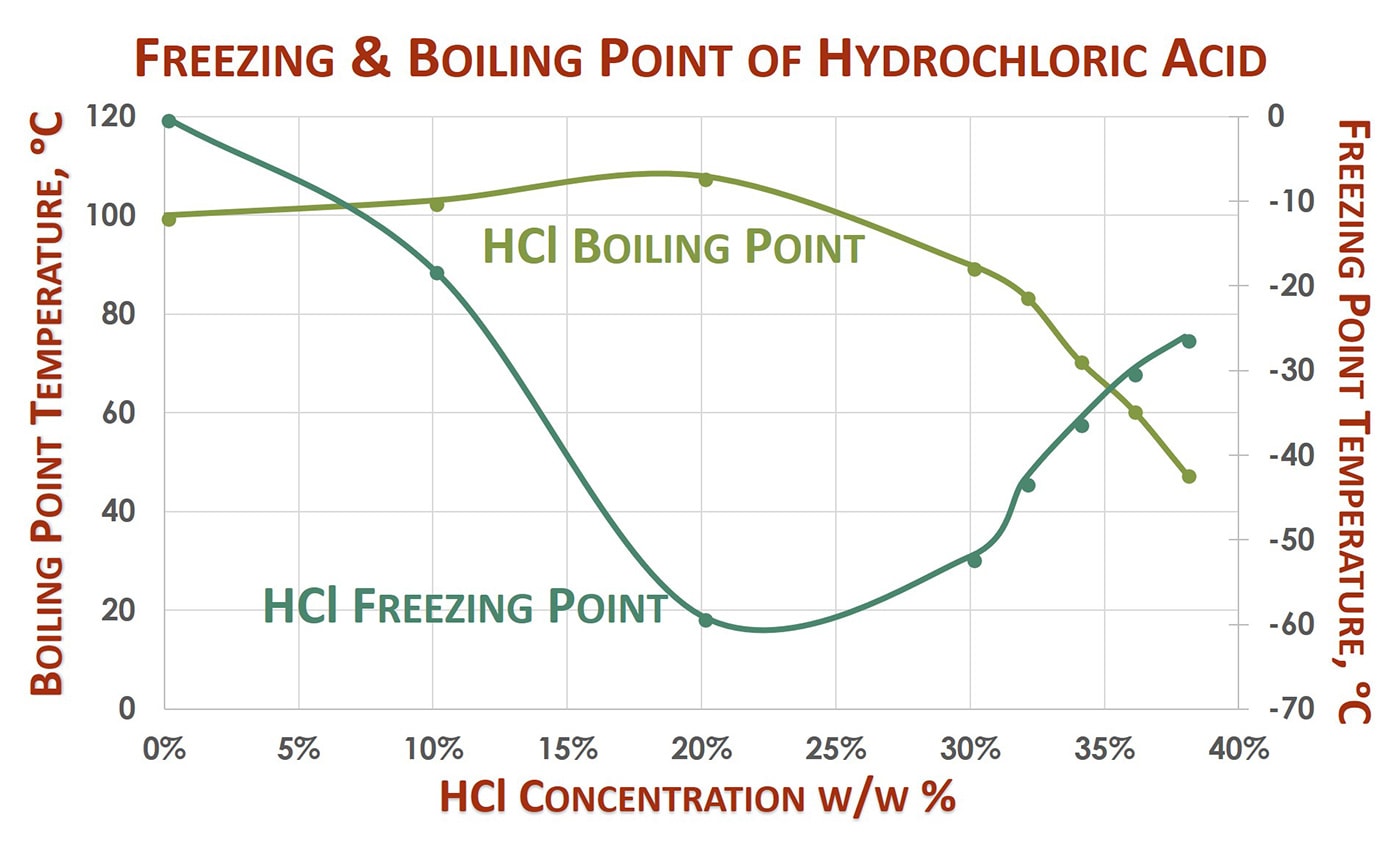
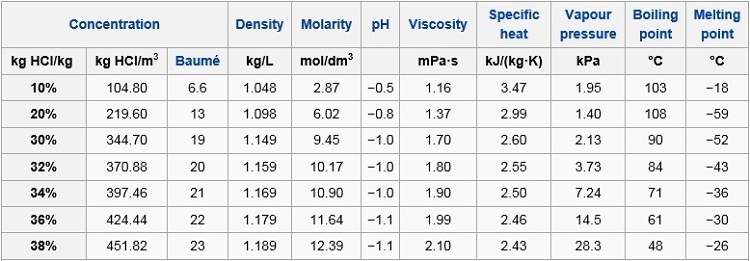
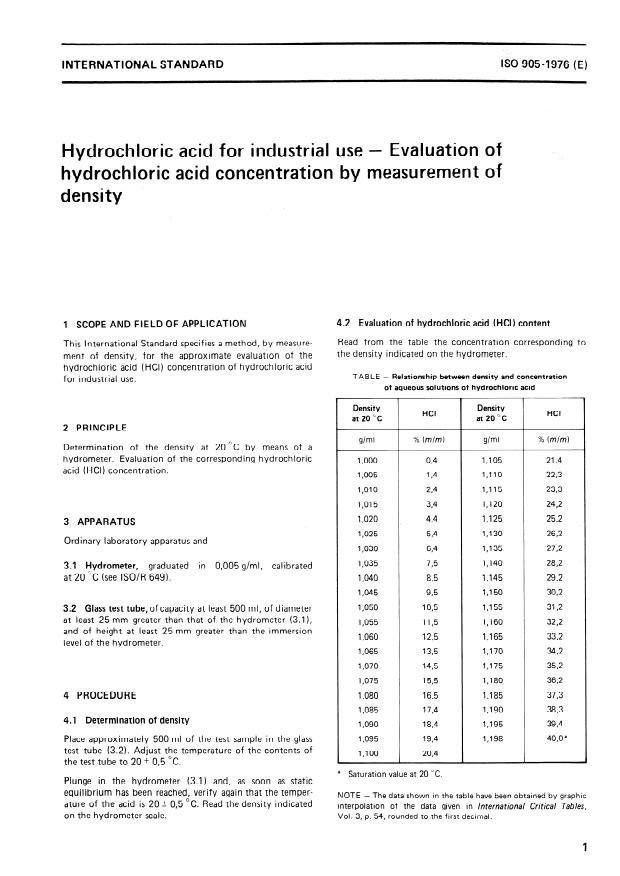
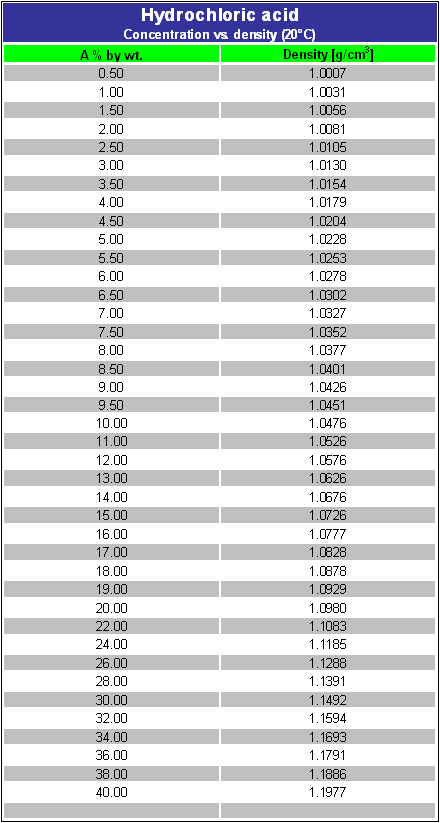
Concentrated hydrochloric acid is `12.0` M and is `36.0%` hydrogen chloride by mass. What is its - YouTube

How to Prepare 1 molar HCl from 37% of HCl having density 1.18 g/cm3. | Umair Khan Academy - YouTube
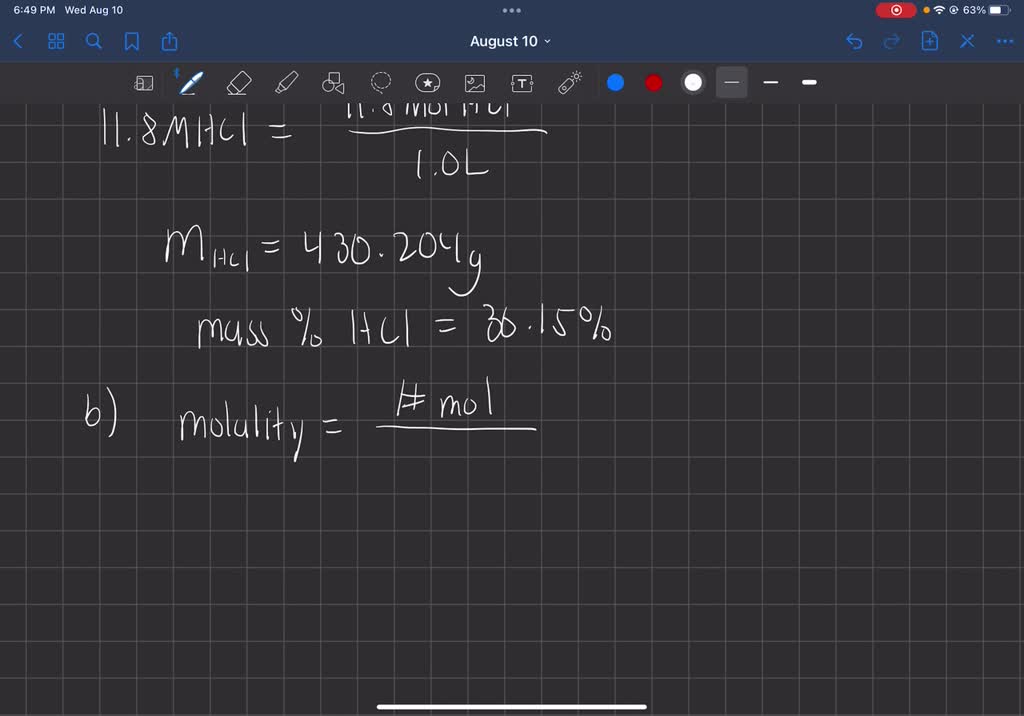
SOLVED: Commercial concentrated Hydrochloric acid is 11.8 M HCl and has a density Of 1.190 g/mL. Calculate the: a. mass percent HCI b. Molality c. mole fraction of HCI

SOLVED: What is the mole fraction of hydrochloric acid in a 7.20% (bY mass) aqueous solution? 0.0383 0.0185 0.0369 The density of the solution is needed to solve the problem: 0.0739